ABOVE: The vaccine protected 90 percent of piglets in a trial from JEV infection. Photo: Australian Pork Limited
Australian Pork Limited is currently funding a project on the development of a novel Japanese encephalitis virus vaccine for pigs.
The vaccine is being developed in conjunction with Dr Jody Hobson-Peters and her team at the University of Queensland.
In 2022, a JEV outbreak caused significant reproduction issues in sows, mainly through mummification and abortion of piglets.
In humans, in rare situations, it can lead to serious neurological symptoms when people are bitten by mosquitoes carrying the disease.
JEV causes significant human health concerns if contracted, with symptoms often becoming permanent or fatal.

Dr Jody Hobson-Peters looked at cells used for the vaccine manufacture in culture with Dr Jessica Harrison.
However, it is important to note that people cannot become infected with the virus through the consumption of pork meat or other pork products.
In pigs that are bitten by infected mosquitoes, the virus is amplified, increasing the risk to humans.
Vaccinating pigs and stopping them from contracting the virus will help to reduce the number of pigs being infected by the virus and thus help mitigate the risk to humans.
The UQ-developed vaccine makes use of chimera virus technology, developing a ‘hybrid’ version of the virus using a harmless-to-humans mosquito-only Australian virus – the ‘Binjari’ virus.
This hybrid virus looks identical to JEV but can only grow in mosquito cells.
Safety and efficacy trials recently undertaken on pigs at the Elizabeth MacArthur Agriculture Institute in NSW have seen the vaccine perform extremely well in generating antibodies and providing immunity.
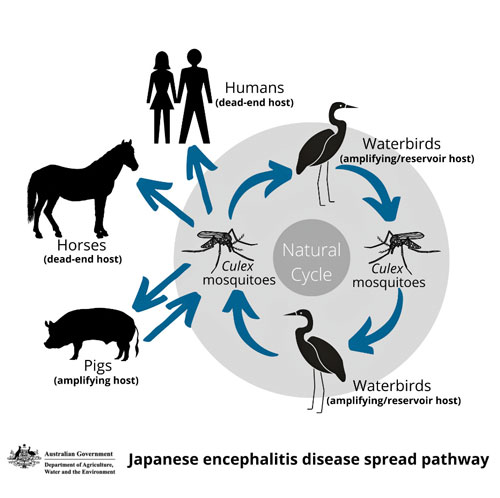
In pigs that are bitten by infected mosquitoes, the virus is amplified, increasing the risk to humans.
In weaners, two doses of the vaccine were 90 percent effective in protecting the pigs from JEV infection.
Additional safety trials in sows will also be conducted to determine the effects of the vaccine in sows.
UQ is currently working with Sydney veterinary vaccine company Treidlia Biovet in manufacturing of the vaccine.
Once passed through the regulatory process, a larger safety and efficacy trial in the field involving several hundred animals will be undertaken.
Pending a successful outcome, it is hoped that a commercially available vaccine will be ready to launch in late 2023.
Dr Raymond Chia
APL Research and Innovation







